



Replication, coverage vary among MD vaccination programs for long-lived birds
Marek’s disease (MD) vaccination programs for breeders and layers can vary significantly in the replication and coverage they provide,1 a finding that should be considered when planning an MD-control strategy for long-lived birds, advises Eduardo Muniz, DVM, PhD, Zoetis.
“All commercially available MD vaccines prevent mortality and the development of tumor lesions, but our study indicates that in long-lived birds, the herpesvirus of turkey (HVT) fraction of a conventional combination MD vaccine may provide better replication and coverage compared to the recombinant vector vaccines,” Muniz said. “In addition, the results indicate that not all CVI-988 vaccines behave the same.”
Protecting commercial breeders and layers against MD in Brazil has traditionally depended on using a nonpathogenic HVT strain along with a Rispens CVI-988 strain,2 he said. Some producers also add an infectious bursal disease (IBD) immune-complex vaccine to their protocol.
More recently, HVT recombinant vector vaccines that protect against MD as well as IBD have become available. They provide convenience for producers since they protect against two diseases. Zoetis is among the animal health companies developing a line of recombinant vector vaccines, which it has already introduced in the US.
Options compared
“Although there is an appropriate time and place for using each type of commercial MD vaccine, we wanted to compare these different options and evaluate their performance specifically in Brazilian breeders and layers,” he said. The study also aimed to determine if adding an immune-complex IBD vaccine would interfere with HVT or CVI replication in the MD vaccines.
For the study, 300 day-old female birds were divided into groups of 100 that were vaccinated against MD post-hatch by subcutaneous injection. Each group received one of the following protocols:
- Group 1: A recombinant vectored HVT IBD vaccine combined with a conventional Rispens CVI-988 strain of MD
- Group 2: A conventional combination vaccine with HVT and the Rispens CVI-988 MD strain (Poulvac® Ovoline CVI+HVT), plus an immune-complex IBD vaccine with the V877 strain (Magniplex®)
- Group 3: A different recombinant vectored HVT IBD vaccine plus a different Rispens CVI-988 strain of MD compared to group 1
The investigators evaluated vaccine replication in 10 pulp-rich feather-tip samples taken from each group at 14, 21 and 28 days of age. The samples were sent to a laboratory where they were tested by real-time polymerase chain reaction (PCR) for both the HVT and CVI components of the MD virus strains in the vaccines. The PCR assays amplified and quantified sequences of genes specific for CVI serotype 1 and for HVT serotype 3.
Unbiased results
Results from the study, sponsored by Zoetis and conducted in partnership with Hendrix Genetics, were unbiased because the investigators evaluating the results did not know which vaccine group the samples were from, Muniz noted.
In general, Poulvac Ovoline CVI+HVT provided the best replication and coverage. For instance, for samples taken at 14 days of age, the log quantifying CVI was significantly higher for Group 2 samples than for Group 3 samples. At 28 days of age, the CVI log was significantly lower for Group 3 compared to Groups 1 and 2 (Table 1), said Muniz, a co-author of the published study.
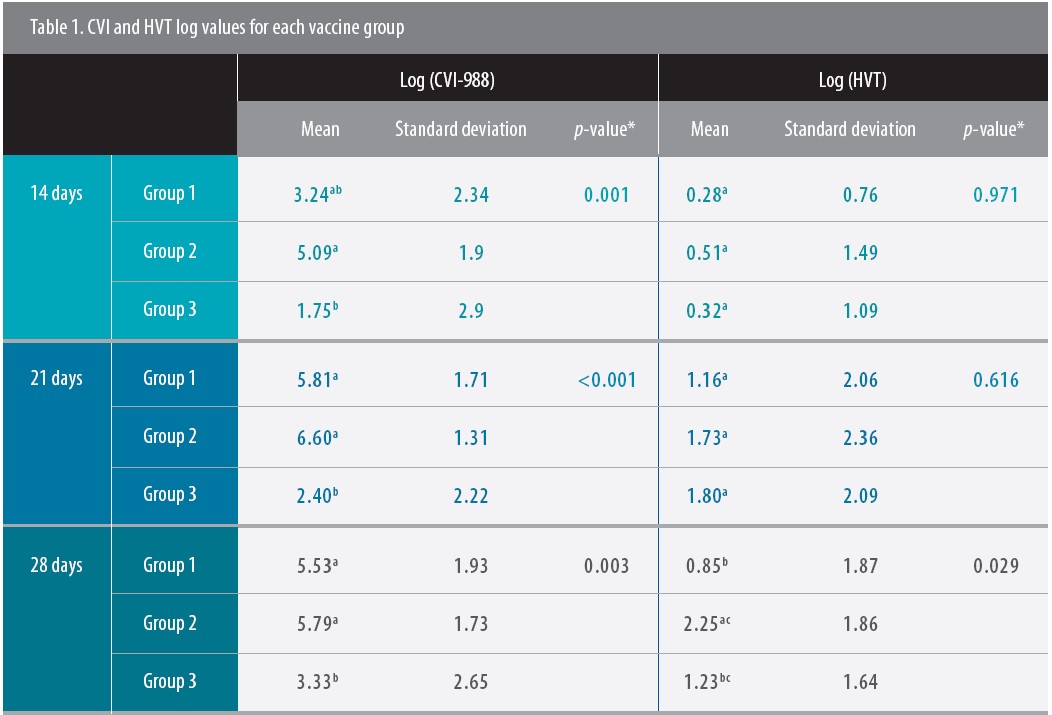
Click on table to enlarge
Feather tips, he explained, are well established as an excellent source for detecting and quantifying MD virus,3 and sampling is easy and non-invasive. The optimum age for evaluating vaccine take is between 2 and 5 weeks post-vaccination, when feather tips are pulp-rich for viral DNA extraction.4
Vaccine efficacy, Muniz continued, correlates with the load, plateau and persistence of the vaccine virus in lymphoid tissue and in feather tips.5 The delay of MD vaccine in reaching its plateau indicates a delay in the establishment of immunity, which would be a disadvantage if birds are exposed to a virulent field strain during the first weeks of life. Conversely, faster replication of the CVI component of an MD vaccine would be an advantage if early exposure occurs.
Replication ‘clearly faster’
“The CVI component of Poulvac Ovoline CVI+HVT presented higher replication levels and had already reached its peak plateau at 21 days. Replication was clearly faster compared to the other groups,” he said.
Replication of the HVT component of Poulvac Ovoline CVI+HVT was slower and less intense than the vaccine’s CVI component, Muniz noted. However, significant differences between groups were observed when the HVT components of the various vaccines were analyzed separately.
The study results also indicate that different vaccination programs should not be based solely and exclusively on vaccine titers, he emphasized.
Vaccine titers, as measured by plaque-forming units (PFU) per dose, were lower for Poulvac Ovoline CVI+HVT, yet the HVT and CVI replication rates were still higher and faster compared to the other groups. In chickens, high PFU titers therefore may not correlate to vaccine performance, he said.
Furthermore, the study found that despite adding an IBD immune-complex vaccine to the conventional vaccine protocol, CVI and HVT replication with Poulvac Ovoline CVI+HVT was still faster than with the other vaccines tested. According to Muniz, this is consistent with study findings from the Philippines indicating that administration of an immune-complex vaccine does not interfere with vectored vaccine efficacy (see sidebar).6
Disease pressure should guide vaccine protocol Exactly which vaccine protocol to use in long-lived birds needs to be guided by disease pressure, Muniz stressed.
“If there is significant disease pressure for MD and IBD, I would want to use Poulvac Ovoline CVI+HVT plus the immune-complex vaccine in laying hens and breeders. The greater replication of the HVT and CVI fractions in this study reinforces my recommendation in this regard,” he said.
“There are more options than ever before for control of MD and IBD. With surveillance of circulating serotypes and careful selection and administration of the vaccines, poultry producers have the capability for excellent control of both diseases.”
| References | ||||
|---|---|---|---|---|
| 1 Muniz EC, et al. Comparison of CVI and HVT vaccine strains replication in feather tips in different Marek’s Disease vaccination programs. ARS Veterinaria, Jaboticabal, SP. 2020;36(4):278-285. | ||||
| 2 Gimeno IM, et al. Evaluation of vaccine strain CVI-988 against Marek’s disease in meat-type chickens. Avian Dis. 2015;59:400-409. | ||||
| 3 Baigent SJ, et al. Absolute quantitation of Marek’s disease virus genome copy number in chickens feather and lymphocyte samples using real-time PCR. J Virol Methodol. 2005;23:53-64. | ||||
| 4 Ibid. | ||||
| 5 Baigent SJ, et al. Development and composition of lymphoid lesions in the spleens of Marek’s disease virus-infected chickens: associations with virus spread and the pathogenesis of Marek’s disease. Avian Pathol. 1999;28:287-300. | ||||
| 6 Villalobos JM, et al. Serological response in commercial pullets vaccinated concurrently with immune complex IBD (V877 strain) and recombinant HVT-ND vaccines. World Vet Poult Assoc. Bangkok, 2019. | ||||













