



Regulatory Mechanism of Response to CpG-ODN, a Pathogen-Derived Molecule, in Chicken Macrophages
The current study expands the possibilities for vaccine development studies by discovering disease response pathways, according to Ceren Ciraci and Susan J. Lamont in Iowa State University Animal Industry Report 2011.Summary and Implications
The regulation and mechanism of action of the avian-specific Toll-like receptors (TLR) genes have been characterized in chicken macrophages in the present study. Chicken TLR15 is a novel pattern recognition receptor whose cognate ligand is unknown and is structurally different from other TLRs. Screening various stimulants in macrophage cell cultures, we concluded that TLR15, in collaboration with TLR2 and TLR21, is involved in CpG oligonucleotide (ODN) response in chicken macrophages. We further verified CpG responsiveness by using inhibitor ODN in macrophage cells. Additionally, we determined the pathway use of MyD88 adaptor molecule after stimulation with CpG-ODNs, resulting in IL1B expression. The current study expands the possibilities for vaccine development studies by discovering disease response pathways.
Introduction
The innate immune response in chickens is critical to clear pathogens and to prime the adaptive immune response. Macrophages are key components of innate immune response and mediate their functions via specialized receptors. The extracellular ligand-binding domain of TLRs (TLR2, TLR3, TLR4, TLR9 and TLR7) immediately recognizes pathogen associated molecular patterns including lipoprotein, double-stranded RNA, lipopolysaccharide, unmethylated CpG, and single-stranded RNA. There are essential differences in the TLR repertoires of mammals and Aves. While TLR15 does not exist in mammals, TLR9 does not exist in Aves. Not only the members of the TLR gene family, but also the regulatory mechanisms of TLRs differ across species. Mammalian TLR4 uses both MyD88-dependent and -independent pathway, but chicken TLR4 does not. To date, no chicken TLR has been reported to use both pathways.
Materials and Methods
We used the chicken macrophage cell line HD11 as a model. Cells were cultured at 41°C and 5 per cent CO2 and treated with several doses of pathogen derived molecules that stimulate TLRs (PAM3CSK4, E.coli and S.enteritidis-derived LPS, double-stranded RNA, imidazoqualinoline, A, B and C type CpG-ODNs) for 3 and 24 hours. Expression of several cytokines (IL10, IL1B, IFNA) and TLRs (TLR15, TLR2, TLR21, TLR4) and 28s genes were measured by quantitative PCR. The standard curves for all tested genes were prepared using serial dilutions of templates. C(t) values were calculated by normalizing to 28s housekeeping gene. Comparisons within dose and time were ranked by Tukey HSD test to define the optimum concentration for stimulants to induce an immune response. P-values were considered significant at P ≤ 0.05. The effect of inhibitor ODN on CpG-ODN stimulated chicken macrophages was tested by quantitative PCR. We designed siRNAs directed at the MyD88 gene. After optimization of transfection conditions, we introduced the siRNAs to macrophage cells using lipofectamine RNAiMAX. Transfection efficiency was calculated by the average number of cells that received fluorescent siRNAs from seven different fields under a fluorescent microscope.
Results and Discussion
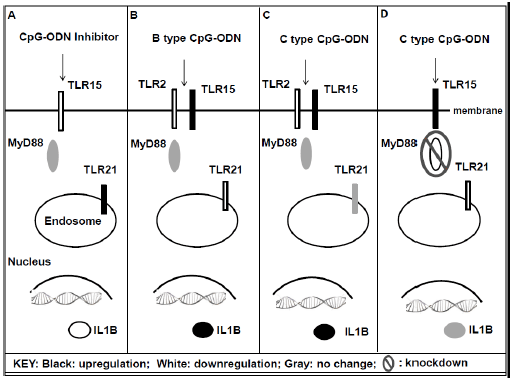
TLR15 was significantly upregulated after induction with both B and C type CpG-ODN, PAM3CSK4, E.coli and S.enteritidis-derived LPS in macrophage cells at 3 hours post stimulation (P < 0.05). To validate the responsiveness of TLR15 to CpG-ODN induction, we measured the effect of CpG-ODN inhibitor on expression of TLR15, TLR21, IL1B, IFNA, IL6 and IL10 by QPCR. While TLR15 and IL1B were downregulated after inhibitor treatment (P < 0.05), TLR21 was upregulated. IL1B consistently had the same gene expression response as TLR15 in macrophage cells after CpG-ODN induction (P < 0.05). Our results suggest that responsiveness to different types of CpG-ODN in chicken macrophages requires multiple receptors and each receptor indicates variation in their expressions. To explore the mechanism of action of CpG-ODN response in chicken macrophages, we examined MyD88 dependency of TLR15 and TLR21. Macrophage cells simultaneously transfected with three siRNAs showed 70 per cent decrease in the expression of MyD88 mRNA compared to negative siRNA transfected macrophage cells (P = 0.03). Although the IL1B gene was significantly induced in CpG-ODN stimulated cells with intact MyD88, this induction disappeared in cells that showed a 70 per cent reduction in MyD88 gene expression (Figure.1). The present study provides the first evidence of MyD88 dependency of TLR signaling in chicken macrophages. Thus, we present evidence of the commonality in the mode of action of CpG-ODN responsiveness in mammals and Aves.
Further Reading
| - | You can view other reports in Iowa State University's Animal Industry Report 2011 by clicking here. |
March 2011











