



Major Viral Diseases of Waterfowl and Their Control
The most common viral diseases in domestic waterfowl species are described by Professor Vilmos Palya, Director of Scientific Support and Investigation Laboratory of Ceva-Phylaxia Veterinary Biologicals Co.in Budapest, Hungary in a paper presented by the British Veterinary Poultry Association (BVPA).Although chickens and turkeys comprise the majority of poultry species seen by the veterinary practitioner, occasionally waterfowl such as ducks and geese are encountered. One feature that is unique to waterfowl is that their environment usually involves the presence of man-made or natural bodies of water, and this may enhance the co-mingling of free-living waterfowl species with the domestic flock and ultimately, promote disease transmission. Additionally, environmental conditions may also influence disease manifestation in domestic waterfowl.
There are many diseases that can affect waterfowl species. The infectious diseases described here are the most common viral diseases seen in domestic waterfowl species. It seems likely that viral diseases will assume greater future importance as causes of disease in waterfowl. Greater attention needs to be given to the study of this source of disease.
Duck Virus Hepatitis
Duck hepatitis (DH) is a highly fatal, contagious and rapidly spreading disease of young ducklings from one to 28 days of age. So far, three different viruses, duck hepatitis virus (DHV) type 1, 2 and 3, have been associated with these disease conditions. DHV-1 has, since the first outbreak in 1949 in Long Island, been reported to infect ducklings worldwide and is of most economic importance to all duck-growing farms because of high potential mortality when infection is not controlled. Molecular characterisation of the DHV-1 genome recently showed that the genome organization classifies this virus as unassigned species in the family Picornaviridae. DHV types 2 and 3 are recognised as separate entities because they induce hepatitis in DHV type 1-immune ducklings, they are now classified as member of the Astroviridae family. Recently, in Taiwan and Korea, new serotypes of duck hepatitis virus, belonging to the same virus family as DHV-1 have been described, which showed no antigenic relationship with DHV-1 in cross-neutralization test.

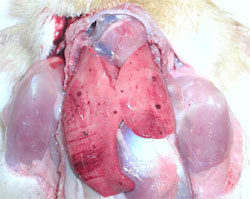
Ducklings are most susceptible to DHV at younger ages and gradually become more resistance as they grow older. The disease is rarely seen in ducklings over four weeks of age. The onset of the disease is very rapid, it spreads quickly through the flock and may cause up to 90 per cent mortality. Sick ducklings develop spasmodic contractions of their legs and die within an hour in a typical “arched-backward” position (Figure 1). The liver is enlarged and shows haemorrhagic spots (Figure 2).
Diagnosis aiming to distinguish between infections caused by DHV-1, DHV-2 and DHV-3 has been regarded difficult by gross and microscopic examination. Recently developed one-step or multi-plex reverse transcriptase-polymerase chain reaction (RT-PCR) methods are capable to detect and distinguish between the different DHV types including the new variant DHV-1 type as well. Typing of duck hepatitis viruses is important to identify emerging serotypes because immunisation is serotype-specific and does not confer protection against infection with heterologous serotypes.
To prevent the disease, keep age groups isolated particularly during the first five weeks of life. Contact with wild waterfowl should be avoided. Rats have been reported as a reservoir of the virus, therefore pest control is important. Vaccination of breeder ducks with an attenuated live duck virus hepatitis vaccine, using type 1 virus, provides maternal immunity that effectively prevents high losses in young ducklings. The vaccine is administered by the subcutaneous route in the neck to breeder ducks two or three times before the birds come into lay and thereafter, every 12 weeks during the laying period. At least three immunisations are advisable for adequate passive protection of ducklings. Inactivated DHV-1 vaccine for use in breeder ducks that have been previously primed with live vaccine has also been described.
Modified live DHV-1 vaccine also can be used for early vaccination of progeny of non-immune breeders. The vaccine is administered by the subcutaneous route or by foot web stab in a single dose to day-old ducklings. The birds rapidly develop an active immunity within three to four days. Hyperimmune serum to DHV-1, prepared from the egg yolk of hyperimmunised chickens, applied by SC in the neck, at the time of the onset of the disease, is an effective treatment of affected flock.
Duck Plague (Duck Virus Enteritis)
Duck virus enteritis (DVE) is an acute, sometimes chronic, contagious virus infection that occurs naturally only in ducks, geese and swans, all members of the family Anatidae of the order Anseriformes. In duck-producing areas of the world where the disease has been reported, DEV has produced significant economic losses in domestic and wild waterfowl due to mortality and decreased egg production. The aetiological agent, a herpesvirus, is a member of the alpha-herpesvirinae subfamily of the Herpesviridae family.
This disease is most likely to affect mature ducks but DVE has been reported in birds ranging from seven days of age to mature breeders. In susceptible flocks, the first signs are often sudden, high and persistent mortality with a significant drop in egg production. In chronically infected partially immune flocks, only occasional deaths occur. Recovered birds may be carriers and may shed the virus in the faeces over a period of years. Clinical signs and gross pathology associated with a DVE outbreak vary with the species, age and sex of the affected birds, and the virulence of the virus. The range of signs in affected birds includes sudden loss of appetite, ataxia, watery diarrhoea and nasal discharge. In ducklings two to seven weeks of age, losses may be lower than in older birds and the signs associated with DVE infection include dehydration, loss of weight and blood-stained vents. The gross lesions are characterised by vascular damage, with tissue haemorrhages and diphtheroid lesions of the mucosal surfaces of the digestive tract. Eruptive lesions of the mucous lining of the oesophagus and intestine are characteristic signs of DVE. Necrotic plaques may be observed in the cloaca. Microscopic lesions are characterised by vascular damage and its consequences in visceral organs. Eosinophilic intranuclear inclusions and cytoplasmic inclusions in epithelial cells of the digestive tract are typically present.
A live attenuated virus vaccine can be used to control DVE in birds more than two weeks of age. Fattening or breeding ducks may be vaccinated subcutaneously or intramuscularly to produce an active immunity. The vaccine virus is not thought to spread by contact from vaccinated to unvaccinated ducks, as the unvaccinated birds remain susceptible to infection. An inactivated vaccine has been reported to be as efficacious as modified live vaccine. This vaccine has been tested only under laboratory conditions; it has not been tested on a large scale and is not licensed.
Reovirus Infection of Muscovy Duck and Goose
Although avian orthoreoviruses have been isolated from different pathological entities of poultry, their pathological role has been confirmed only in a limited number of diseases, such as arthritis-tenosynovitis of chickens and stunting runting syndrome. A disease of Muscovy ducks caused by reovirus was first described in South Africa in 1950, then in France in 1972, where the virus was isolated. Reoviruses have been repeatedly isolated from geese and detection of antibodies to reoviruses has also been reported. However, diseases caused by a reovirus in this species was reported only in 2003.
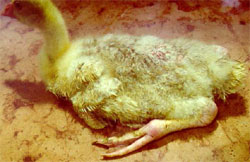
The earliest onset of the disease, both in duck and goose flocks, is between seven and 10 days of age and may persist in an affected flock until seven to 10 weeks of age. The outbreaks last for two to four weeks or even longer. Morbidity ranges from 10 to 60 per cent, and mortality from two to 20 per cent. Mortality is always higher in young flocks (two to three weeks of age) than if infection occurs in older birds. The clinical signs in the acute phase include a general malaise, accompanied by diarrhoea of sick birds. The affected birds are reluctant to move when disturbed. Muscovy ducks and goslings that survives the acute phase of the disease are markedly stunted in growth and especially geese develop lameness. The hock and metatarsal or digital joints, as well as the gastrocnemius and digital flexor tendons, and sometimes the synovial bursae are markedly swollen (Figure 3).
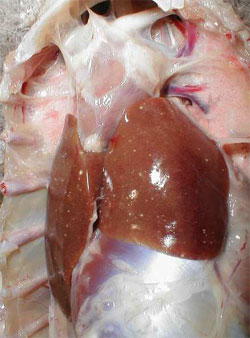
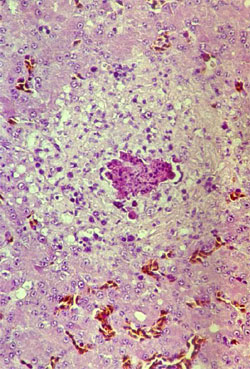
By post mortem examination, during the acute phase of the disease, characteristic lesions can be seen in the liver and spleen: in both organs, multiple disseminated, greyish-white pin-head necrotic foci are present and they are larger than normal (Figure 4). Sero-fibrinous epi- and pericarditis, arthritis and tenosynovitis are frequently seen during the acute and chronic phase of the disease. As a consequence of the rupture of the tendon and surrounding tissues, large haemorrhages in the region of gastrocnemius flexor tendon are observed in the chronic phase of the disease. By histology, miliary foci of necrotic hepatocytes or granuloma-like foci with necrotic centres and proliferating macrophages can be found in the liver and the spleen (Figure 5).
Diagnosis of the disease can be based on the characteristic liver and spleen lesions during the acute phase and on the development of arthritis/tenosynovitis during the subacute-chronic phase. Classical detection methods of the causative virus involves the isolation of the virus in reovirus antibody negative duck/goose embryo or embryo liver cell cultures and detection by electron microscopy, all which are laborious and time-consuming.
Recently, however, a rapid, sensitive and brod-spectrum RT-PCR has become available for the detection and identification of avian reoviruses from cell cultures and clinical samples. It is important to confirm the identity of reovirus isolates upon an outbreak since despite the common properties shared between duck/geese and chicken reoviruses, the two viruses are antigenically different and their core protein coding genes show only 21 to 25 per cent homology at nucleotide and amino acid levels.
Although reovirus disease of Muscovy duck and goose continues to cause heavy losses to the Muscovy duck and goose industry, specific prevention of the disease has not been developed. Field attempts to protect with an inactivated duck reovirus vaccine were unsuccessful, despite the promising experimental results. A live vaccine prepared from non-pathogenic or attenuated reovirus did not induce immunity or protection. A subunit vaccine consisting of baculovirus-expressed major capsid protein was found non-immunogenic.
Parvovirus Infection of Waterfowl
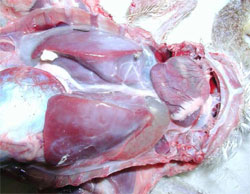
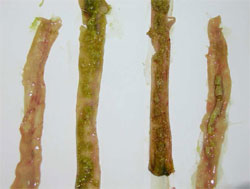

Waterfowl parvoviruses – goose parvovirus (GPV) and Muscovy duck parvovirus (MDPV) – cause serious disease in goslings and Muscovy ducklings. Occasionally, the disease accounts for mortality of 70 to 100 per cent in susceptible flocks when the infection occurs at early age of life. GPV and MDPV differ in host range and antigenicity, while geese are fully resistant to MDPV infection, in Muscovy ducks both viruses can cause severe disease.
It has been shown that, even if a certain level of antigenic relation exists, there is a clear distinction between GPV and MDPV. Cross-protection studies carried out in maternal antibody-free, susceptible Muscovy ducklings indicated that only bivalent vaccine containing both goose and Muscovy duck parvovirus antigens provide adequate clinical protection against the two waterfowl parvoviruses that can cause disease in Muscovy ducks. In addition, in mule duck (cross-breed of Pekin duck and Muscovy duck) the so-called ‘short beak and dwarfism syndrome’ has been reported where animals had strong growth retardation with smaller beak and shorter tarsus.
The diseases caused by waterfowl parvoviruses are strictly age-dependent. In susceptible goslings and Muscovy ducklings under one week of age, 100 per cent mortality may occur, while the losses above this age are decreasing with the age. In birds with impaired immune system, the infection may cause significant economic losses up to six to eight weeks of age. Depending on the age when infection occurs, the disease may be present in either acute, sub-acute or chronic forms in goose and Muscovy duck, while SBDC of mule duck always takes the chronic form.
During the acute phase of the disease, infected animals excrete huge quantity of virus into the environment with their faeces, which disseminates the infection rapidly in the flock. Recovered animals or those infected at a later age can become healthy carriers. Due to its resistance in the environment, parvovirus can persist in the buildings and on poorly cleaned and disinfected surfaces, which results in transmission to subsequent flocks. Vertical transmission and egg shell contamination also plays an important role in introducing the infection into disease-free flocks.
Parvovirus infects rapidly dividing cells; this is why clinical form of the disease occurs only in young birds, up to approximately six weeks of age. Nevertheless, infection with other immunosuppressive viruses (reovirus, cicrovirus) and mycoplasmas tends to aggravate the clinical disease by their synergistic effect and prolongs the sensitivity period to the clinical manifestation of the disease up to nine weeks of age. After this susceptible period, the birds can still be infected which causes serological response without clinical symptoms. The disease may be complicated with secondary bacterial pathogens: E. coli, Streptococcus spp, Pasteurellas etc.
| Common clinical signs and pathological lesions in parvovirus infection | |
| Clinical signs: | Pathological lesions: |
|---|---|
Acute form:
|
Acute form:
|
Chronic form:
|
Chronic form:
|
Clinical diagnosis is insufficient, especially during the chronic phase of the disease, and laboratory confirmation is necessary. PCR and serology are the most commonly used laboratory tests for the confirmation of the clinical diagnosis.
Control
The specificities of parvovirus infection of waterfowl require the elaboration of a coherent and efficacious vaccination strategy. The optimal vaccination strategy must take into account the presence or absence of maternal antibodies, their levels and heterogeneity within a flock and the susceptible period of goslings and ducklings to the disease.
Breeder geese and Muscovy ducks that have been naturally infected or vaccinated transfer maternal antibodies via the egg yolk to their progeny. This passively acquired antibody may persist until two to four weeks of age depending on the day-old antibody levels of individual birds. Since the disease is confined to young geese and Muscovy ducks, control measures have been aiming at providing adequate immunity during the first six to eight weeks of life. To achieve this, different methods have been applied during the last three decades. These include: (i) passive immunisation of newly hatched birds with convalescence or hyperimmune serum, (ii) active immunisation of adult breeding geese and Muscovy ducks with virulent virus and inactivated vaccine, and (iii) the use of attenuated vaccine alone or in combination with inactivated one for the active immunisation of both adult and young animals. The attenuated vaccines can confer good protection in young animals but only when it is given to birds with no or very low level of maternally derived antibodies to parvoviruses.
Historically, hyperimmune or convalescence serum injected subcutaneously in day-old goslings was used to avoid heavy losses in flock exposed to a parvovirus-contaminated environment. This technique was effective but presented the risk of carrying over undetected infectious agents by the contaminated serum. Therefore, serum for controlling the diseases is hardly available any more and the prophylaxis is based on vaccination.
Two main categories of vaccines can be distinguished: live and inactivated vaccines. Live vaccines contain attenuated goose parvovirus, which can stimulate rapid immune response and protection in maternal antibody-free animals. MDA, even at a very low level, is able to neutralise the live vaccine thus preventing it to stimulate immune-response. Inactivated vaccines (also called ‘killed’) contain the whole parvovirus antigens either in the monovalent (goose parvovirus) or bivalent (both goose and Muscovy duck parvovirus) form.
Most of the parvovirus vaccines available on the market are attenuated live vaccine and contain only goose parvovirus. The advantage of live attenuated vaccines is the fast onset of immunity in susceptible birds, however, the induction of immunity by live vaccines is dependent on the presence of circulating maternal antibodies to parvoviruses at the time of vaccination. When using live vaccine at an early age, it is a regular observation that the presence of passively acquired antibodies interferes with the development of an active immune response. On the other hand, inactivated vaccines with high antigen content are able to induce active immunity and protection in face of maternal antibodies. The disadvantage of the inactivated vaccine is the relatively slow immune response.
The immunisation of breeders against parvovirus has two objectives:
- to protect the breeders from infection, and by this way prevent virus transmission to the progeny, and
- to supply the progeny with passive immunity.
Breeders transmit protective maternally derived antibodies (MDA) to the offspring through the egg yolk. The level of MDA determines the level of protection (morbidity and mortality) of goslings and ducklings in case of field virus infection. Knowing the level of maternal antibodies in the day-old birds is fundamental to establish adequate vaccination strategy. A positive correlation has been demonstrated between the number of immunisations of breeders with inactivated vaccine and the amount of transmitted maternal antibodies, including level of MDA, persistence of MDA in the young birds and protection offered by the passive immunity. MDA may persist in goslings and ducklings at a relatively high level which protects them from clinical disease for approximately two to three weeks. Moreover, MDA levels after vaccination of breeders with traditional live vaccines show an inevitable heterogeneity (low/high MDA).
Optimal vaccination strategy must protect goslings and ducklings in all their life against both the early and the late forms of the disease. In order to extend the protection after the maternal antibodies decline to un-protective level, the vaccination of goslings and ducklings before they reach seven to 10 days of age is essential to stimulate active immunity in face of still persisting maternal antibodies. To achieve a stronger and more durable immune response, a booster vaccination (around two to three weeks of age) is also recommended. These can be done only by the use of inactivated, high antigen content vaccine.
Hemorrhagic Nephritis Enteritis Virus Infection of Geese (HNEG)
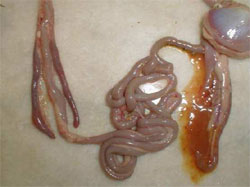
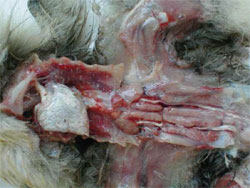
HNEG is characterised by high morbidity and mortality rates in geese from three to 10 weeks of age. Since its first report in 1969, several outbreaks with an epizootic pattern have been reported in almost all major goose-breeding countries. Although HNEG has been well characterised at the clinical level and recognised as a viral disease many years ago, its causative agent remained unknown until 2000, when evidence was presented that the disease is caused by a polyomavirus. Based on phylogenetic analysis, it was concluded that the causative agent of HNEG is closely related to but clearly distinct from other polyomaviruses, likely representing a distinct virus species named 'goose hemorrhagic polyomavirus'.
The majority of the outbreaks occur between three and six weeks age but sometimes much younger (four days old) or older birds (17 to 20 weeks old) can be affected. The mortality can vary within a huge range (four to 67 per cent) and can continue for extended periods (one to two months), or with an interruption of several weeks, i.e. two separate mortality peaks could be observed.
Animals in infected flocks generally develop normally and then suddenly die with no premonitory signs. Other animals develop clinical signs that include ataxia, tremors of the head and neck, subcutaneous haemorrhages and the excretion of blood-stained faeces. However, once the clinical signs develop, the animals die rapidly. Geese, which recover from HNEG are supposed to be persistently infected.
The most frequent and characteristic gross and histopathological lesions are summarized in Table 2 and Figures 9 and 10.
| Table 2. Frequency of characteristic gross and histopathological lesions in HNEG | ||
| acute cases | Subacute cases | |
|---|---|---|
| Oedema in the subcutaneous tissues and ascites | +++a | ++ |
| Hydropericardium | ++ | ++ |
| Hemorrhages in the subcutaneous tissues | +++ | -/+ |
| Hemorrhages in the brain | ++ | - |
| Anaemia | + | ++ |
| Catarrhal enteritis | ++ | ++ |
| Hemorrhagic enteritis | +++ | -/+ |
| Liver degeneration | ++ | ++ |
| Zonal hemorrhagic tubulonecrosis | +++ | +++ |
| Visceral gout | - | -/++ |
| a: + = slight, ++ = moderate, +++ = marked, - = negative | ||
Since the isolation of GHPV in many cases is very difficult or not possible, the PCR test for the detection of polyomavirus nucleic acid is the only practically available method to confirm diagnosis on an aetiological basis. The GHPV-specific DNA could be detected in various organ samples including kidney, liver, spleen, lung, bursa of Fabricius and intestinal contents from natural cases of the disease.
Commercial vaccine against GHPV is not yet available, partially due to the difficulties in the propagation of the GHPV in embryonated eggs and cell cultures. Therefore, the focus of GHPV vaccine development turned to sub-unit vaccines. The immunogenic antigen VP1 of GHPV was recently successfully expressed in both insect cells and yeast. It was also demonstrated that in both expression systems, VP1 alone or in combination with VP2, forms virus-like particles (VLP), and this as an antigen could be used in the development of vaccines and serological tests.
Circovirus Infection of Geese and Ducks
Relatively little is known about the diseases with which avian member of the genus Circovirus are associated. Avian circovirus infections show certain common features. They are seen in birds during the first months of life. Developmental and/or feathering disorders predominate the clinical signs. Damage to the lymphoreticular tissue is expected to impair both humoral and cellular immune functions. Circovirus-induced immunosuppression enhances the pathogenicity of co-infecting agents. The course and outcome of the infection depend on the concurrent infections present and other predisposing factors. Sub-clinical infections seem to occur that may cause considerable economic loss. One common future of circovirus infection is that these viruses invade lymphoid tissue that may lead to immunosuppression.
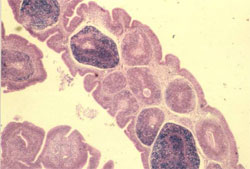
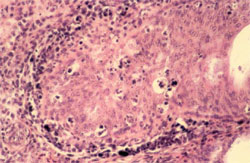
Circovirus infection of geese and ducks was first described in Germany by Soike et al. (1999) and Hattermann et al. (2003), respectively. The commercial goose and duck farms, in which circovirus was diagnosed, exhibited growth retardation, feathering disorders and increased mortality due to secondary infections with Riemerella anatipestifer and Aspergillus fumigatus.
The main histological changes associated with circovirus infection in waterfowl species are those of the primary and secondary lymphoid tissues. They are commonly observed in the bursa of Fabricius (BF) and may range from lymphofollicular hyperplasia to lymphoid necrosis, lymhocytes depletion and cystic atrophy (Figure 11). The frequent detection of globular or botryoid, basophilic intracytoplasmic inclusions within macrophages appears to be a characteristic feature (Figure 12).
Circovirus infections are diagnosed on the basis of feathering abnormalities, histology of BF, and demonstration of virus antigen or nucleic acid. Knowledge of the genome sequences of goose and duck circoviruses allowed the development of diagnostic tests such as PCR. Molecular epidemiological results to date indicate that different avian species are infected by different circoviruses, suggesting that circoviruses are host-specific.
Attempts to prevent and control diseases caused by Circovirus are very limited. Given that infections with circoviruses are likely to be prevalent and these viruses are highly resistant to inactivation, eradication is unlikely to be regarded as an option for disease control. On the grounds that for vaccine manufacturing purposes, an efficient antigen production system is required and that circoviruses cannot be grown by conventional culturing method and are difficult to inactivate, it is reasonable to expect that a sub-unit vaccine based on the expression of virus protein by recombinant DNA-technology would be the target for vaccine development.
Further Reading
| - | Find out more information on the diseases mentioned in this article by clicking here. |
June 2011












