Three research-proven ways to maximise Marek’s disease control
For decades, vaccination has provided effective control of Marek’s disease (MD). Since the first MD vaccines were introduced in 1970, broiler condemnation losses due to MD have decreased more than 99%. [1]By Tarsicio Villalobos, DVM, Zoetis
However, the emergence of new and more virulent MD serotypes over the years has required vaccination strategies to evolve as well. In the past, for example, turkey herpesvirus (HVT) and bivalent vaccines (a combination of serotype-3 HVT and serotype-2 SB-1) provided adequate protection. Now they are both still necessary, but not always sufficient, depending on the MD virus (MDV) strain.
Unfortunately, MD remains one of the most significant viral diseases of poultry worldwide, causing an estimated $1-2 billion in losses each year. [2] The good news, however, is that vaccination remains a highly effective method of controlling it, even as vaccination strategies continue to evolve. Following are three research-proven tips for keeping your flocks protected against this potentially devastating disease:
Include CV988 strain in your MD vaccination program
Like virulent MD strains, MD vaccine strains are grouped under three different serotypes: serotype 1 (CVI988), serotype 2 (SB-1) and serotype 3 (HVT). Currently, the serotype-1 MDV strain CVI988 offers the greatest protection against virulent MD, especially when used in combination with other vaccines. In a 2015 study, vaccine protocols that included CVI988 were found to provide numerically better protection against the highly virulent MDV strain 648A than protocols that used only vaccines of serotypes 2 (SB-1) and 3 (rHVT) (Figure 1).[3]
Figure 1. Protocols with CVI988 and/or in ovo provide best protection against early challenge
Thus, including CVI988 in your vaccination program is one important way to ensure adequate MD protection. However, the same study reveals that disease control is linked not only to the type of vaccine, but also to the method of administration: protocols that included in ovo vaccination consistently provided better protection than ones in which the vaccines were administered at hatch.
Vaccinate in ovo
These findings are consistent with previous studies showing that all commercially available MD vaccines offer better protection when administered in ovo compared to subcutaneous vaccination at hatch.[4]
In a 2001 study, a CVI988 vaccine was tested to determine if it induces early post-hatch protection against MD. In one group of chickens, the vaccine was injected in ovo on embryonation day (ED) 17, and in a second group, it was administered subcutaneously at hatch. At 1, 2, 3, 4, 5 and 7 days of age, chickens from each group were exposed intra-abdominally to MDV RB1B, a virulent serotype-1 MDV variant, and sampled to determine MDV lesions. Results indicate that in the first 4 days after hatch, the in ovo-vaccinated chickens were significantly better protected against virulent MDV than those vaccinated at hatch (P < 0.001) — an important benefit in view of the very young age at which chicks are first exposed to the virus (Figure 2).[5]
Figure 2. In ovo vaccination provides better MD protection
Another study on the optimisation of double vaccination protocols produced similar findings more than a decade later — a significant finding in view of MD’s evolutionary tendency towards greater virulence.
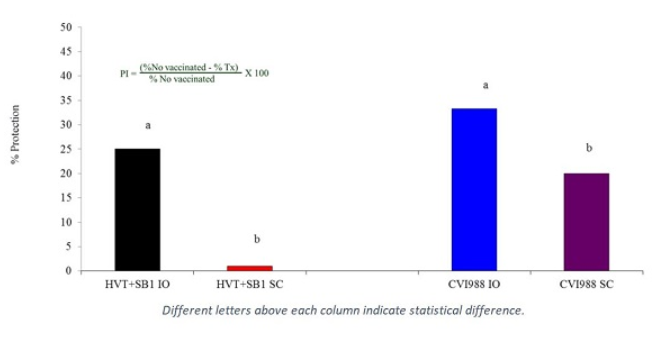
In this study, broiler chickens were given the same CVI988 and bivalent (HVT+SB1) vaccine combinations either in ovo at ED 18 or subcutaneously at hatch, and then challenged at 2 days of age with highly virulent MDV 648A. Once again, in ovo vaccine application was found to induce significantly higher levels of MD protection than subcutaneous injection (P < 0.05) (Figure 3). [6]
Figure 3. In ovo vaccination at ED 18 (IO) vs. subcutaneous (SC) vaccination at hatch, with MDV challenge at day 2
Use HVT vaccines to promote immune development
While CVI988 offers the greatest protection against virulent MD, HVT vaccines remain important tools for MD management. For more than 40 years, they have been used successfully to control MD, both alone and in combination with other vaccines, but recent research shows that they may also contribute to the embryonic chicken’s immune development.
A study was conducted to determine the effect of in ovo administration of a commercially available HVT vaccine on the maturation of the embryonic immune system. Following vaccination on ED 18, researchers evaluated splenic cell phenotypes at 1 day of age and the ability of the day-old chickens to respond to various antigens compared with older birds.
Results demonstrate that day-old chickens that had received HVT in ovo had higher percentages of several lymphocyte lineages in the spleen than day-old sham-inoculated chickens (1-day sham) (Figure 4). Furthermore, in ovo administration of HVT rendered chicks at hatch more responsive to unrelated antigens, such as concavalin A and keyhole limpet hemocyanin. These findings suggest that it is possible to accelerate the maturation of the embryonic immune system by administering HVT in ovo.
Figure 4. Immunophenotypes in spleen
Summary
Vaccination remains the best defence against the potentially devastating effects of MD, but as new and more virulent serotypes have emerged, vaccination strategies have had to keep pace. Several studies show that in ovo vaccination with any of the commercially available MD vaccines has a positive impact on vaccine performance, with protocols including CVI988 vaccines offering the greatest MD protection. Furthermore, in ovo administration of HVT vaccines has been shown to have a positive impact on the immune development of embryonic chickens, an important benefit in view of the well-documented immunosuppressive effects of MD. [7] These findings suggest that in ovo vaccination programs that include vaccines of all MDV serotypes (CVI988, SB1 and HVT) may offer optimal protection against MDV as the virus continues evolving toward greater virulence.
References:
1. Gimeno IM. Marek’s disease vaccines; A solution for today but a worry for tomorrow. Vaccine. 2008;26(s3):C31-C41.
2. Marek’s disease virus. The Pirbright Institute. Available at https://www.pirbright.ac.uk/viruses/mareks-disease-virus.
3. Avian Dis. 2015;59:400-409
4. Producers should consult local product labels to determine if in ovo administration is licensed in their country.
5. Zhang Y, Sharma JM. Early Posthatch Protection Against Marek’s Disease in Chickens Vaccinated In Ovo with a CVI988 Serotype 1 Vaccine. Avian Dis. 2001;45(3):639-641.
6. Gimeno IM, Cortes AL, Witter RL, Pandiri AR. Optimization of the Protocols for Double Vaccination Against Marek’s Disease by Using Commercially Available Vaccines: Evaluation of Protection, Vaccine Replication, and Activation of T Cells. Avian Dis. 2012;56(2):295-305.
7. Faiz NM, Cortes AL, Guy JS, et al. Early infection with Marek’s disease virus can jeopardize protection conferred by laryngotracheitis vaccines: a method to study MDV-induced immunosuppression. Avian Pathol. 2016;45(6):606-615.