Highly Pathogenic Avian Influenza A/H5N1 – update and overview of 2006
By the European Centre for Disease Surveillance and Control - As of 29 November 2006, 258 human H5N1 infections that meet its strict laboratory criteria have been reported to the World Health Organization (WHO) since reporting began for 2003.Avian influenza 2006: human situation
Of these 258 cases, 154 patients have died (60%) and there has been no decline in that high mortality rate over time [1,2]. There has been a disproportionate concentration of infections in children and young adults, even allowing for the relatively young populations in the ten countries where human infections have occurred, and there is an over-representation of females among patients aged 10-29 years [2]. This is thought to be related to the fact that it is usually young people and women who look after domestic poultry.
There is some evidence of familial clustering which may suggest a genetic susceptibility [3,4]. Asymptomatic and mild infections do occur but appear to be very rare, although more sero-epidemiology around confirmed cases is needed to confirm this impression [3-6]. In the second half of 2006, there was a steep decline in the number of case reports, although similar declines occurred in 2004 and 2005, but were then followed by resurgences (Figure 1) [1,2].Critically, human to human transmission, as indicated by cluster size, is still extremely inefficient, as it was a decade ago when the first human to human transmission took place in Hong Kong [3-5].
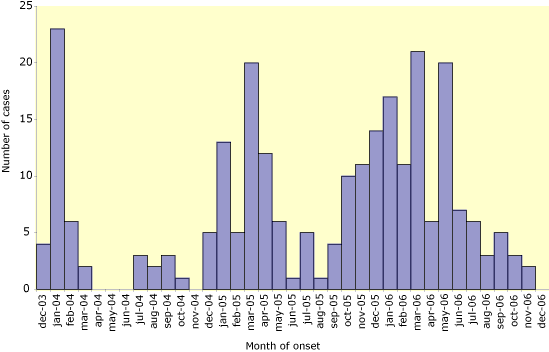
Figure 1. No. of confirmed human cases of H5N1 infection reported to
WHO by month of onset, 1 December 2003 – 11 December 2006
Animals still source of human infections
In 2003, highly pathogenic avian influenza viruses type A/H5N1 (Asian strain) re-emerged and spread rapidly, infecting poultry and some humans in a number of southeast Asian countries, particularly Vietnam, Thailand, Cambodia and Indonesia [7]. The mechanism for this spread remains unclear although it is suspected that it was as much related to trade of poultry and poultry products as the movements of wild birds. An exceptional multi-species epizootic at Qinghai Lake in northwest China in May 2005 seemed to demonstrate a role of wild birds in the spread of the viruses beyond Asia [8]. From Qinghai, the virus spread to Central Asia, Europe and some African countries with human cases reported in Turkey, Iraq, Azerbaijan, Djibouti and Egypt [1,2,7]. Now, at the end of 2006, the virus has been confirmed in birds in over 50 countries, with birds (almost entirely domestic poultry) being the source of human infections in ten of these [2,7,9].
Some countries are facing up to endemic infection in their national poultry flock and consequent ongoing risks to humans with domestic poultry, while others are barely affected. At a recent world conference on avian influenza and pandemic preparedness [footnote], field reports on efforts to control avian influenza were presented by national and international authorities. There is evidence that H5N1 viruses have now become entrenched in backyard poultry in Indonesia, and perhaps also Egypt [10,11]. Large scale programmes of poultry immunisation have been underway in China and Vietnam where, since 2005 and until an outbreak in the Mekong Delta this week in Vietnam [12], poultry outbreaks had stopped being reported [9]. The scale of immunisation in China, with potentially 14 billion poultry needing to be vaccinated twice annually (in spring and autumn), is the largest immunisation programme against avian influenza ever attempted anywhere in the world.
In the European Union (EU), the virus has not become established in poultry nor have there been there any human infections even though the virus was found in wild birds in at least fifteen countries in the spring of 2006 (Figure 2). Some cats and a pine marten that fed on infected birds were also infected [13]. The bird movements to the EU may have been exceptional following an unusually cold spell of weather in Russia and Central Asia in early 2006. After the spring wave, there have only been confirmation of sporadic H5N1 infections in birds in Spain and Germany (Figure 2).
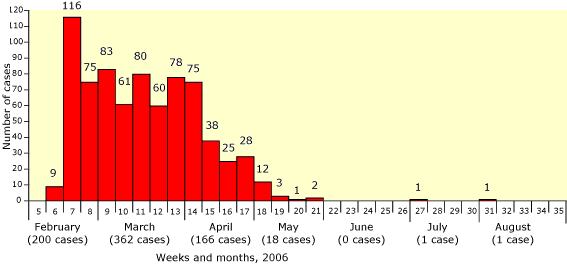
Figure 2. Highly pathogenic avian influenza cases in wild birds in the EU member states notified to the European Commission in 2006.
The successful protection of domestic birds in EU countries was primarily due the robust and consistent application of veterinary measures directed under EU legislation. As a consequence, only five poultry outbreaks occurred in the EU and these were rapidly contained [14,15]. However, the continuing sporadic reports demonstrate that the virus may still sometimes be present and therefore, routine biosecurity measures and early warning systems cannot be relaxed. There were major outbreaks of infection in wild birds and domestic poultry in the Danube delta in 2005 and 2006, and the Romanian authorities successfully contained these. There will be an additional challenge for EU authorities if it occurs here again after Romania joins the European Union next month.
Continuing evolution of the viruses
There remains the risk of emergence of a human pandemic strain through either mutation of the H5N1 virus or incorporation of part of its genome, through recombination, into a human influenza virus [7,16]. As well as extending their range geographically the H5N1 viruses have diversified genetically into clades and sub-clades. Clade 1 dominated in 2003-2004, then clade 2 became more important. Clade 2 has subsequently developed into three distinct sub-clades [7,17,18]. The balance between the types of virus continues to change, for reasons that are not clear.
For example, since 2005, the Fujian-like virus (clade 2, sub-clade 3) has become the dominant type found in surveillance of market poultry across southern China [17]. Fortunately, despite genetic changes, there has been no evidence of significant change in the viruses’ effects on humans. The genetic differences and the fact that the virus is continuing to change are, however, important considerations since the clades have different antiviral resistance profiles and continuing genetic change will alter the necessary composition of human H5N1 vaccines referred to as ‘pre-pandemic vaccines’ [7,18]. Two countries have already committed to purchasing these vaccines and others are considering to do so, although it is by no means clear that an H5 based pandemic is inevitable[7,16].
Discussion
There are many important unknown factors relating to the spread of H5N1, including the current distribution of the viruses. The pattern of H5N1 infection in Africa remains elusive because surveillance is especially weak there, apart from Egypt and some parts of Nigeria [11,19]. The picture is also incomplete in eastern Asia - following two human cases in summer 2006, the situation has improved in Thailand, but the risk remains [20]
A good picture of the zoonotic situation in China is currently not available and it is also still unclear whether the H5N1 vaccination programmes in China and Vietnam have been successful in eliminating or just reducing the level of infection in poultry, and whether low levels of circulating viruses pose a significant human risk [7,21] One negative consequence of any success of vaccination programmes is that surveillance for sporadic human cases is made more difficult, since now, when atypical pneumonias occur, there is rarely the marker of local poultry deaths to inform decisions on whether to test the patient for H5N1 virus.
The relative role of the commercial movement of animals and wild birds in the international spread and local distribution of H5N1 viruses remains controversial. However, it is local preparedness and response that are most crucial in determining the outcome in terms of domestic animal and human health when countries are challenged by the virus. Nationally organised veterinary services, which would enable effective surveillance/early warning and biosecurity systems, are crucial so that authorities can respond promptly when infections are first suspected in either birds or humans. Where biosecurity is poor and veterinary services ineffective, viruses can become endemic and the situation can be complicated by the virus cycling between poultry and wild birds [10,11,17].
One challenge developing countries face is a lack of financial support for the veterinary services and biosecurity measures, even though avian influenza has demonstrated that it is truly an international problem. There has been some progress towards a solution for the financial issues by the involvement of the World Bank, the European Commission and the United Nations System Influenza Coordinator (http://www.undg.org/content.cfm?id=1482), which have mobilised and released donations that had been pledged by national and international donors [22].
The data indicate that H5N1 avian viruses remain poorly adapted to humans. With a high enough viral challenge and perhaps some genetic host susceptibility the viruses can infect humans, in which case they are then often lethally pathogenic, although they are still unable to transmit efficiently between humans [2-5,16]. The H5N1 viruses have been around for nearly a decade and it might be tempting to conclude that if they were going to proceed to form or contribute to a pandemic strain, they would have done so by now. However, it should be remembered that it is thought that the avian influenza virus which contributed to the 1918-19 ‘Spanish Influenza’ H1N1 pandemic strain had been around for some years before it became part of a virus that could efficiently transmit between humans and so be a successful pandemic strain [23].
Apart from the threat from H5N1 there are still many issues around influenza pandemic preparedness (irrespective of the virus type) which need urgent attention. One key area is how authorities in developing countries should best focus their efforts with preparedness, given often very limited resources and many more immediate competing priorities. So far, most discussion, ideas and research have been more suited to settings in better resourced nations. This area needs a multi-sector approach as medical services will not have the most to offer in poorer countries when it comes to preparing for a pandemic. It is hoped that the next world meeting, planned for New Delhi in late 2007 (and intervening technical meetings), will provide opportunities to tackle preparedness in developing nations as well as dealing with avian influenza.
Footnote. The Bamako conference organised by the African Union, the Interafrican Bureau for Animal Resources and the European Union. International Conference on Avian and Human Pandemic Influenza, (Ministerial Meeting and Pledging Conference) 6-8 December 2006, Bamako. Documentation and presentations at the conference are viewable at : http://www.avianinfluenzaconference4.org/index.php?id=61
December 2006