How Do Poultry Adaptive Immune Systems Protect Birds from Harm?
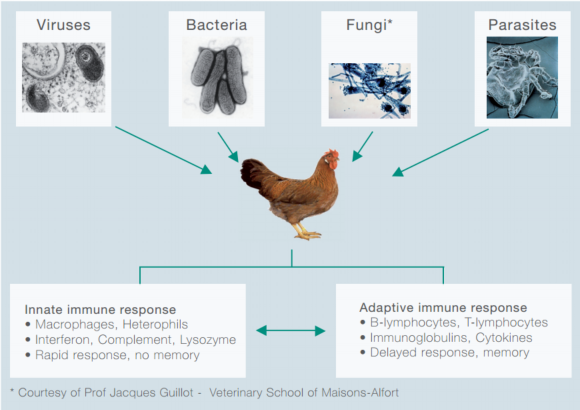
Professor Bernd Kaspers, University of Munich, discusses the health threats poultry face, and aspects of the adaptive immune system that helps to fight off infection.Birds are constantly challenged by a diverse set of pathogens such as viruses, bacteria, protozoa, helminthes and ecto-parasites (Figure 1).
Figure 1: Types of poultry pathogens and immune systems.
These micro-organisms have developed unique and divers strategies to invade the host, to replicate and to evade the defence system.
On the other side, the immune system has co-evolved as a complex “organ” with multiple defence mechanisms to respond appropriately to the plethora of pathogens.
It is composed of a rapid and a delayed response system, called the innate and the adaptive immune system, respectively.
Frequently, the innate immune system is sufficient to control infections but, if this is not the case, the adaptive immune system will become activated. It should be noted that both systems closely interact; examples of this cooperation will be presented later.
The adaptive immune system is composed of B- and T-lymphocytes which share a unique property: the ability to memorise previous contact with pathogens and to recall this encounter during reinfection, thus mounting a rapid and highly efficient immune response.
This short paper will focus on the B-lymphocyte system. First, a brief review of the structure and function of antibodies, the primary secretory product of B-cells, will be presented. Next, the development of B-cells during embryogenesis and the role of the bursa of Fabricius will be discussed.
Finally, it will be explained how B-cells are activated to generate antibodies and how immunological memory is achieved. The role of T-lymphocytes will only be discussed in the context of T-cell help for B-cells.
The antibody
Antibodies are proteins which are produced by B-cells and plasma cells and secreted into the tissues and blood (1). Because of their globular structure they have also been named immunoglobulins.
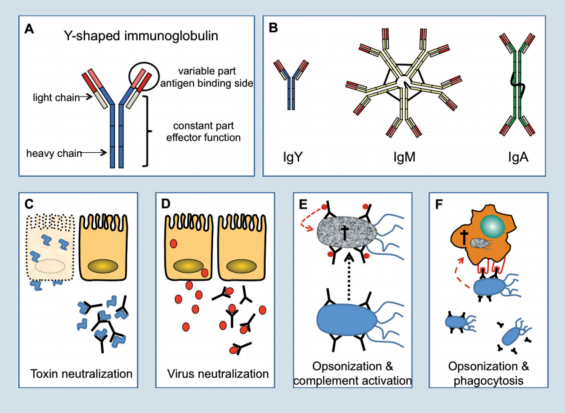
In chickens three types of immunoglobulins are present, which are IgM, IgY and IgA (figure 2). Birds lack a homologue of the mammalian IgE molecule.
The basic structure of all immunoglobulins is Y-shaped and composed of two smaller (light chain) and two larger (heavy chain) proteins linked together by disulfi de bonds (Figure 2A).
On one end the immunoglobulin molecule can bind antigen while the other end mediates important effector functions once the antigen is bound.
The first immunoglobulin produced during an immune response is IgM (Figure 2B). In this molecule five of the basic Y-shaped units are fused to form a pentamer, which allows the molecule to bind multiple antigens at the same time.
IgM is largely found in the blood and to a minor degree as a secreted protein on mucosal surfaces.
During the course of a primary infection, and in response to secondary infections, IgY becomes the most prominent immunoglobulin. IgY is a monomer and secreted at high concentrations into the blood (7-12 mg/ml), but it is found at comparatively low amounts on mucosal surfaces.
During egg yolk formation, IgY is actively transported from the hens blood into the yolk. As the developing embryo absorbs the yolk content, IgY is transferred into the embryonic circulation to protect the chicken during the first critical time after hatch.
In contrast to IgM and IgY, IgA is found in small quantities in the blood. Nevertheless, IgA is the most abundant immunoglobulin in the body, since it is produced in very large quantities by B-cells of the mucosal tissues and is directly secreted as a dimer or trimer onto the mucosal surface to prevent microorganisms from entering.
Figure 2. Types of antibodies and how they work.
Antibodies act in many different ways to control infections. However, their activity is largely restricted to the extracellular space. Intracellular pathogens are not well controlled by antibodies; examples are viruses while replicating inside the cell and intracellular bacteria such as Salmonella, Listeria or Mycobacteria.
However, in the extra-cellular space and on mucosal surfaces, antibodies can firmly bind to soluble molecules or structural components of micro-organisms.
Binding of antibodies to bacterial or fungal toxins, can neutralise their toxic activity which is best known from anti-tetanus toxin antibodies induced during vaccination (Figure 2C).
In the case of viruses, antibodies can bind to viral antigens during viremia (IgY) or on mucosal surfaces (IgA) and prevent infection of cells through the inhibition of virus-cell interaction (Figure 2D).
For example, antibodies directed against the hemagglutinin of influenza virus act in this way. Binding of antibodies to bacteria can mediate bacterial killing through different pathways.
Coating of bacteria with antibodies is called opsonisation and will lead to complement activation and subsequently to bacterial lysis by the complement components (Figure 2E). Furthermore, coated bacteria are recognised by heterophils and macrophages which will phagocytose and kill the pathogens.
To identify opsonised micro-organisms, phagocytes carry on their cell surface receptors which bind to the antibodies coating the surface of the pathogen (Figure 2F). None of these mechanisms can be compensated effectively in antibody deficient animals. Therefore, antibody deficiency is a life threatening condition.
B-cell development in the bursa of Fabricus
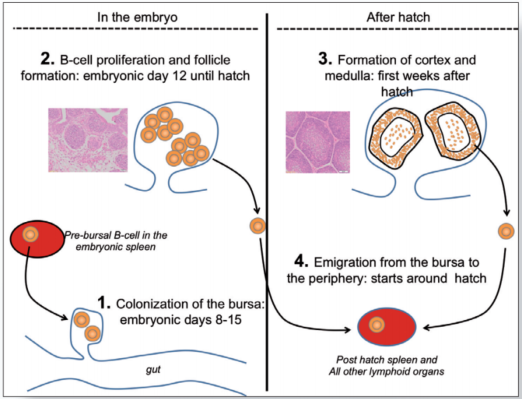
The B-cell system develops during embryogenesis and the first few weeks after hatch. The central organ in this process is the bursa of Fabricius which is connected by an open duct to the proctodeum (figure 3).
It provides the environment for immature B-cells to differentiate into fully functional antibody producing B-lymphocytes (2).
Consequently, surgical removal of the bursa or destruction of the organ by chemicals, toxins or viruses, leads to severe immunosuppression and high susceptibility of the birds to pathogenic challenge, as well known by avian veterinarians.
Between embryonic day (ED) 8 and 15, Pre-bursal B-cell precursors (B-cell “stem cells”) migrate from the embryonic spleen and bone marrow into the bursa (Figure 3.1).
Here they receive signals from the bursal tissue which activate the maturation program. The nature of these signals is still largely unknown. However, the events taking place in the developing B-cells in response to these signals, have been studied intensively.
Initially the bursa is populated by a few thousand pre-bursal progenitors which, as a first step, start to proliferate and to form aggregates of B-cells called bursal follicles (Figure 3.2). It has been estimated that a mature bursa contains up to 12000 follicles with roughly 2 x 105 B-cells per follicle.
During B-cell proliferation, a second important process is initiated, which ultimately leads to the formation of billions of B-lymphocytes, each of them producing a single unique antibody molecule with a unique binding specificity. The molecular mechanism of this process is well known and has been described in detail (3).
Once fully matured, the B-cells leave the bursa and populate the peripheral tissues. This event starts around hatch and is believed to continue until the bursa disappears at sexual maturation of the birds.
However, bursal development is not accomplished at hatch. During the first two weeks after hatch the follicles undergo a structural transformation with the development of a follicular cortex and a medulla (Figure 3.3).
It is believed that the medulla functions as a secondary lymphoid organ very much like the spleen while the cortex generates new B-cells which continue to populate the peripheral lymphoid organs (Figure 3.4).
Surgical removal of the bursa has shown that this organ is of critical importance until chickens are at least at the age of 4-5 weeks.
At that time a population of B-cells starts to appear in the spleen that can maintain the B-cell system of chickens in which the bursa was removed (4). However, the bursa is still required during the following weeks, to guarantee a fully functional B-cell system.
Figure 3. Role of the bursa
B-cell function in secondary lymphoid structures
B-cells leaving the bursa colonise secondary lymphatic structures, in particular the spleen, the caecal tonsils (CT) in the gut, the bronchus associated lymphoid tissue in the lung (BALT) and lymphoid follicles in the mucosal tissues.
Here the cells get in contact with pathogens or with antigen delivered by vaccination. As outlined before, B-cell development in the bursa leads to billions of cells each of them producing an antibody with a different antigen binding side.
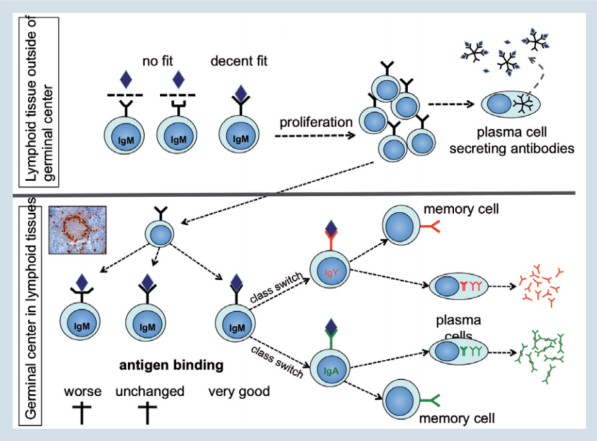
This immunoglobulin molecule is anchored in the cell membrane, with its antigen binding site facing towards the environment (figure 4; upper part).
It is an IgM type immunoglobulin expressed as a monomer and is referred to as the B-cell antigen receptor or BCR. Binding of the proper antigen (an antigen that fits at least decently well) to the BCR, will activate the B-cell and induce its proliferation and differentiation to an antibody secreting plasma cell.
During this process, plasma cells lose their BCR on the cell surface but start to synthesise large amounts of their immunoglobulin molecule and secret it into the tissues and blood. This activation process requires 5-6 days, explaining why the appearance of the first antigen specific IgM antibodies is observed nearly one week after vaccination.
A characteristic feature of the early IgM antibodies, is their low antigen affinity which results from an imperfect fit between the antigen and the binding site of the IgM molecule. This disadvantage is compensated by the pentameric structure of the secreted IgM, which allows simultaneous binding at multiple sites thus increasing the overall binding strength.
Figure 4. Adapting to challenges
Two features of the antibody response have fascinated immunologists for a long time. The first one is the appearance of IgY or IgA antibodies in the course of the response to an infection, a process that is called “immunoglobulin class switch”.
The second one is the production of antibodies with increasingly stronger binding to the antigen, called “affinity maturation”. Both events take place in a well-known structure in lymphoid tissues, the germinal center (GC).
In birds they are round structures found in the spleen, the caecal tonsils and the BALT (Figure 4: photo). After the first encounter of an antigen, some of the proliferating cells will initiate the formation of GCs.
During this process the cells will continue to divide and at the same time will start to mutate the immunoglobulin gene (figure 4; lower part).
These mutations will take place in the antigen binding site and change it in such a way that the binding strength is either unchanged or worsened or, preferably, improved.
Only those B-cells with an improved immunoglobulin molecule will continue to proliferate and, in a next step, will receive signals to change from IgM to IgY or IgA (class switch). Finally, these cells will differentiate to plasma cells and start to secret large amounts of their immunoglobulin.
The final outcome of the process is the generation of antibodies which can “grab” the antigen more strongly and consequently eliminate the pathogen most efficiently.
This is an important mechanism as illustrated by the highly increased susceptibility to infections in patients with a failure in the class switching process. Such defects, albeit rare, have been described in man and mice but also in chicken lines.
It should be noted that this process does not take place in the absence of T-cells. Critical signals for affinity maturation and class switching are provided by the so called T-helper cells.
Consequently, absence of T-helper cells, or functional insufficiency, will cause severe immune deficiency in the B-cell system.
While affinity maturation and class switching lead to improved protection, the formation of memory cells will help animals to respond very rapidly with the secretion of high affinity IgY or IgA antibodies to reinfection.
Memory cells generated in GCs during the primary infection (figure 4), can live for months to years.
In mice and man these cells survive in the bone marrow. Their location in birds is largely unknown but their existence is absolutely certain. Induction of memory cells is the primary aim of vaccination.
Primary vaccination will lead to the generation of memory cells which become reactivated during booster vaccination or in case of infection by the same agent.
This secondary response generates large numbers of B-cells derived from the memory B-cell pool, which produce even larger amounts of antibodies with further increased binding strength. In addition, new memory cells are formed, to be available for a subsequent challenge by the respective pathogen.
References
- Härtle S, Magor K.E., Göbel, T.W., Davison F. Kaspers B. Structure and evolution of avian immunoglobulins. pp 103- 120; In: Avian Immunology, Schat K.A., Kaspers B., Kaiser P. 2nd edition, Elsevier 2013.
- Oláh I, Nagy N. Retrospection to discovery of bursal function and recognition of avian dendritic cells; past and present. Dev Comp Immunol. 2013, 41(3):310-5
- Ratcliffe M.J.H., Härtle, S. B cells, the bursa of Fabricius and the generation of antibody repertoires. pp 65- 90; In: Avian Immunology, Schat K.A., Kaspers B., Kaiser P. 2nd edition, Elsevier 2013.
- Toivanen P, Toivanen A. Bursal and postbursal stem cells in chicken. Functional characteristics. Eur J Immunol. 1973, 3(9):585-95