



The role of xylanase in animal nutrition: mechanisms, types, and innovations
Why is the GH10 family of xylanases more effective?Among the enzymes that play a critical role in optimizing animal nutrition, xylanase is widely acknowledged for its ability to break down complex polysaccharides in plant-based feed ingredients, improving nutrient utilization and overall animal performance. However, in recent decades, despite advances in production, processing technologies, and utilization of by-products, xylanase enzyme technology has lagged behind, especially in flexibility required for modern diet formulation.
The role of Xylanase in animal nutrition
Xylanase targets xylan, a major component of hemicellulose found in plant cell walls. It is commonly used in animal nutrition to enhance the digestibility of dietary fiber, particularly in monogastric animals such as poultry and swine. Plant-based feed ingredients, such as corn, wheat, and soybean meal, are rich in non-starch polysaccharides (NSPs), including arabinoxylans, which are difficult for animals to digest efficiently.
Xylanase helps break down these complex NSPs, releasing more simple sugars and other nutrients for absorption in the digestive tract. This not only improves feed efficiency but also reduces the anti-nutritional effects of NSPs, such as gut viscosity and increased microbial fermentation, which can lead to digestive disorders and reduced animal performance.
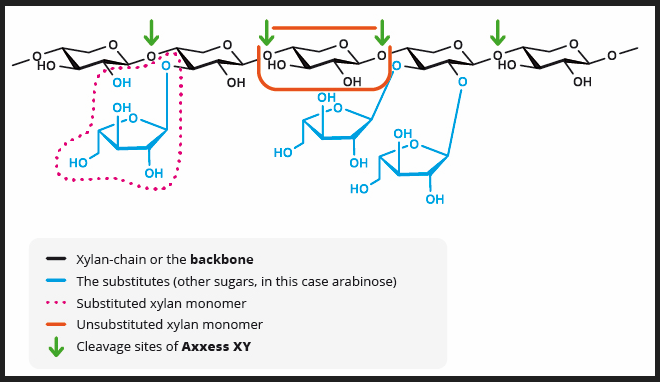
GH10 xylanases make it possible to incorporate higher levels of cost-effective ingredients
While the GH11 family of xylanases, commonly employed in feed, effectively lowers viscosity levels in broiler gastrointestinal tracts by reducing soluble NSPs in wheat-based diets, they prove less effective when confronted with the more complex arabinoxylan backbone of insoluble NSPs.
The 3D structure of GH11 xylanase explains its limitations. GH11 xylanases require 3-4 consecutive unsubstituted xylan monomers on the backbone to locate an active site, making them less effective in the presence of side chains on arabinose backbones. They are highly specific, favoring low-branching wheat backbones.
In contrast, GH10 xylanases, though not commonly used in feed, offer a distinct advantage. They require only two or fewer consecutive unsubstituted xylan monomers to find an active site, enabling them to act on xylose residues near branches. This results in more and shorter xylo-oligomers than GH11 xylanases produce. Simply put, GH10 xylanases have a shallower cleft, providing greater catalytic versatility (Pollet 2010).
This versatility allows for a broader range of feed ingredients, including co- and by-products, while maintaining performance. Therefore, GH10 xylanases make it possible to incorporate higher levels of cost-effective ingredients, presenting a significant opportunity to reduce overall feed costs.
Why is the GH10 family of xylanases more effective?
Substrate Specificity: GH10 xylanases tend to exhibit a broader substrate specificity compared to GH11 xylanases. They can efficiently hydrolyze a wider range of xylan structures found in various plant cell walls. This broader specificity allows for better degradation of different types of dietary fiber present in animal feed ingredients.
Enhanced Thermal Stability: GH10 xylanases often demonstrate better heat resistance, which is crucial in the feed manufacturing process. The ability to withstand higher temperatures during feed processing, such as pelleting or extrusion, ensures that the enzyme remains active and effective throughout the entire production cycle.
Improved pH Tolerance: GH10 xylanases may have better pH tolerance, allowing them to function optimally in the diverse pH conditions of the digestive tract. This can lead to improved enzyme activity and greater efficiency in breaking down xylan in the animal's gastrointestinal system.
Higher Catalytic Efficiency: GH10 xylanases are known for their higher catalytic efficiency, meaning they can break down xylan molecules more rapidly and effectively. This leads to better utilization of nutrients in the feed, improved feed conversion, and enhanced animal performance.
Resistance to Protease Inhibition: In some cases, GH11 xylanases may be inhibited by the presence of proteases or other enzymes in the digestive system. GH10 xylanases are often more resistant to such inhibitory effects, ensuring their continued activity in the gut.
Broader Application Range: Due to their versatility and improved performance under various conditions, GH10 xylanases are better suited for a wide range of feed formulations and animal species, making them a more flexible choice for commercial nutritionists.
Recent innovations in enzyme technology have further improved the effectiveness of xylanase and other enzymes in animal nutrition. Customized enzyme solutions, heat-stable xylanases, enzyme combinations, molecular genetics, and nutrigenomics are all contributing to the advancement of precision nutrition in animal production. As these innovations continue to evolve, they hold the promise of further optimizing animal performance and sustainability in the livestock and poultry industries.












