



US Poultry Industry Manual - Egg safety rules
Learn more about egg safety regulations and egg breaking plantsPart of Series:
< Previous Article in Series Next Article in Series >
Editor's Note: The following content is an excerpt from Poultry Industry Manual: The Foreign Animal Disease Preparedness and Response Plan (FAD PReP)/National Animal Health Emergency Management System (NAHEMS) Guidelines which is designed to provide a framework for dealing with an animal health emergency in the United States. Additional content from the manual will be provided as an article series.
Egg Farms and Laying Hens
Federal regulations concerning laying hens and eggs are written to ensure food safety by preventing contamination of food with particular pathogens, such as Salmonella spp. Regulation of chickens producing eggs for human consumption is the responsibility of the Food and Drug Administration (FDA). On July 9, 2009, FDA published in the Federal Register a final rule entitled “Prevention of Salmonella enteritidis in Shell Eggs During Production, Transportation, and Storage.” The regulation became effective on July 9, 2010 for producers with 50,000 or more laying hens and will become effective on July 9, 2012 for producers with 3,000 or more laying hens. The Egg Safety Rule is intended to reduce Salmonella enteritidis (SE) and requires a) monitoring of pullets, b) minimum biosecurity standards, c) rodent and insect control, d) cleaning and disinfection of poultry houses, e) refrigeration of eggs on the farm (45°F or less), f) environmental monitoring, and g) record keeping.
Shell Eggs
Regulation of shell eggs is shared among various Federal agencies and State governments, or shell eggs may be processed according to industry-generated protocols. Federal authority to regulate egg safety is shared by the Department of Health and Human Services’ (HHS’) Food and Drug Administration (FDA) and the United States Department of Agriculture’s (USDA’s) Food Safety Inspection Service (FSIS). In addition, USDA’s Animal and Plant Health Inspection Service (APHIS) supervises the National Poultry Improvement Plan which certifies poultry breeding stock and hatcheries as SE-free and USDA’s Agricultural Marketing Service (AMS) conducts a surveillance program to ensure proper disposition of restricted shell eggs (Figure 6).
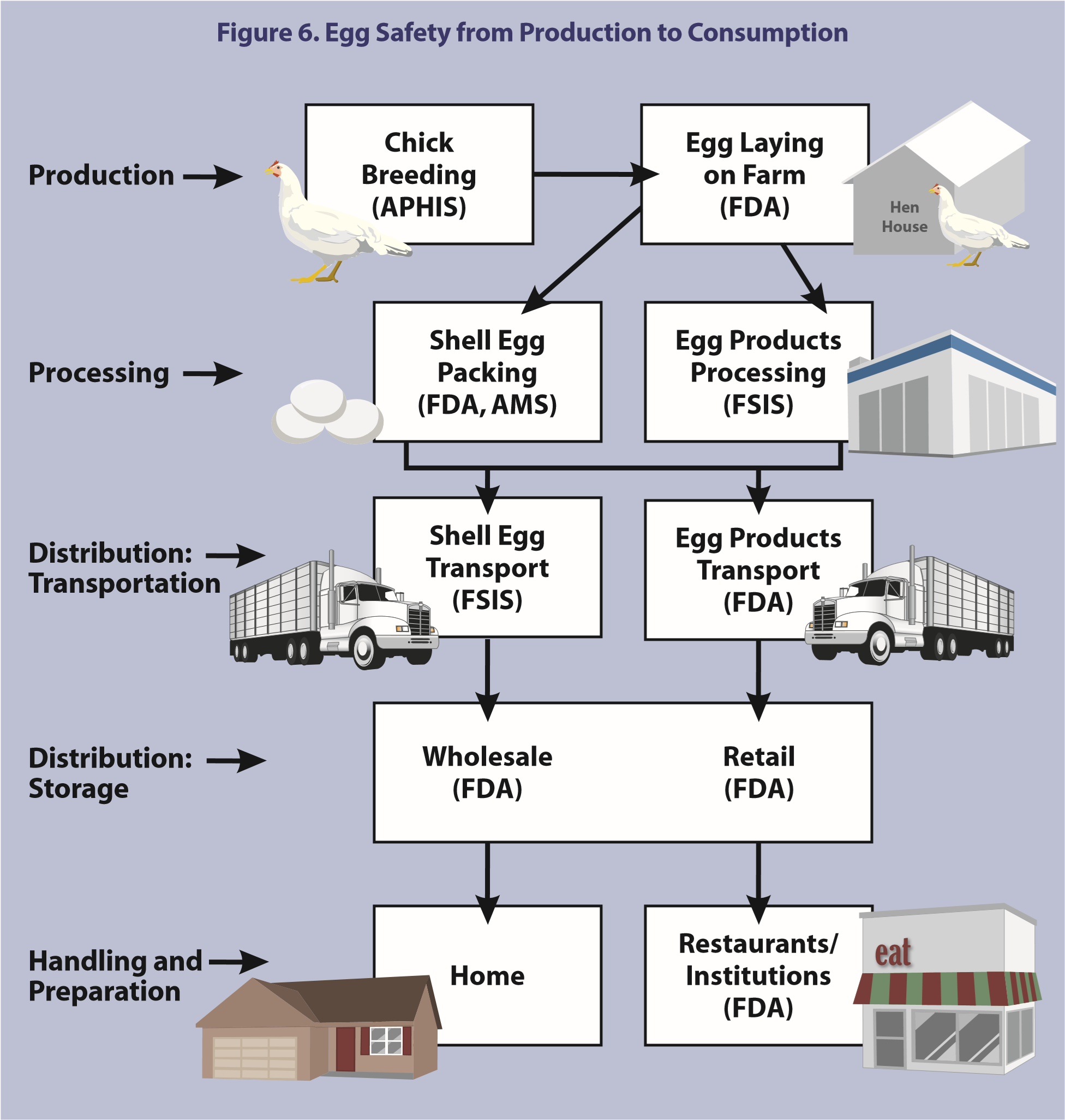
FDA has jurisdiction over the safety of foods generally, including shell eggs, under the Federal Food, Drug, and Cosmetic Act (FFDCA; 21 U.S.C. 301 et seq.). Under the Public Health Service Act (PHSA; 42 U.S.C. 201 et seq.), FDA also has the authority to prevent the spread of communicable diseases, including the authority to regulate foods when the foods may act as a vector of disease, as in the case of SE in eggs. FDA is responsible for: (1) investigating SE outbreaks, reported by CDC and State/local health departments, due to foods in interstate commerce, (2) performing trace backs to identify the source of the implicated eggs, (3) testing flocks, (4) diverting eggs from SE-positive flocks, (5) collecting flock data to help track the spread of SE among layer flocks, and (6) promoting better quality control.
USDA has primary responsibility for implementing the Egg Products Inspection Act (EPIA; 21 U.S.C. 1031 et seq.). Under EPIA, FSIS has primary responsibility for the inspection of processed egg products to prevent the distribution of adulterated or misbranded egg products. The AMS offers a voluntary shell egg grading program (7CFR56) which includes minimum facility sanitation and construction requirements as well as daily oversight of egg wash and rinse water temperatures, sanitizer strength, and cold storage temperatures. Also, AMS oversees a shell egg surveillance program that places limitations on restricted eggs, such as eggs that are cracked, dirty, contain blood, leak fluid, or are inedible (7CFR57). The shell egg surveillance program prevents the movement or sale of adulterated or misbranded eggs for human food. Eggs in cartons bearing the USDA shield have been graded and checked for weight and the carton must include a code which identifies the packing plant and day of packing. Shell egg operations that do not fall under 9CFR590 and choose not to participate in the voluntary AMS shell egg grading program typically adhere to state regulations or follow industry-generated protocols. State regulations vary, and many states rely on industry protocols rather than regulating shell operations at the state level.
Breaking Plants and Egg Products
FSIS is responsible for inspection of egg breaking plants and the inspection of liquid, frozen, and dried egg products used by food manufacturers, food service, institutions, and retail markets. FSIS mandates specific washing and sanitization conditions for eggs prior to breaking (9CFR590) and all plants breaking eggs under 9CFR590.24 are required to have continuous inspection by an FSIS inspector. Inspectors must be onsite whenever breaking equipment is operating unless specifically exempted. FSIS requires a label that indicates refrigeration of egg products is needed.
Egg products may be shipped between official plants as either unpasteurized (raw) or pasteurized egg products in bulk tankers. When bulk shipments of liquid egg products are handled in this manner they are required by regulation [9 CFR 590.410 (b)] to be shipped under USDA seal (cannot be a company seal) and be accompanied with a FSIS PY200, Egg Products Inspection and Grading Certificate. The USDA commodity specification for the egg product requires a tamper evident seal to be applied to tanker shipments. Seals applied to such shipments are normally embossed with an alpha-numeric code. Alpha-numeric codes on seals and proper sealing of tankers are verified by an FSIS inspector prior to shipment. Individual seal codes are documented on USDA certificates accompanying shipments.
Bulk Tanker shipments of pasteurized liquid egg products to non-official outlets (further processors or manufacturers that are not official FSIS plants) are not required to be shipped under USDA seal and certificate. FSIS highly recommends for biosecurity purposes and to maintain the integrity of these shipments that the shipping plant seal/ secure the shipment to maintain product integrity and protect the product against tampering.
Reference: "USDA APHIS | FAD Prep Industry Manuals". Aphis.Usda.Gov. 2013. https://www.aphis.usda.gov/aph...
The manual was produced by the Center for Food Security and Public Health, Iowa State University of Science and Technology, College of Veterinary Medicine, in collaboration with the USDA Animal and Plant Health Inspection Service through a cooperative agreement.















